Helium

Fun Facts About Helium:
- Helium makes up about 24% of the mass of the universe and is the second most abundant element!
- The word helium comes from the Greek helios, which means sun!
- Helium atoms are so light that they are able to escape Earth's gravity!
- Helium that is bought in cylinders (like the ones used to fill your party balloons!) is produced by radioactive decay of thorium and uranium!
Contributor: The University of Toledo Instrumentation Center
What is Helium used for?
About the Display: As one of the noble gases, Helium was chosen by the Instrumentation Center. The display box features a discharge tube in the shape of the element's symbol. The tube is filled with Helium and powered by a transformer. When an electric charge excites the Helium gas in the tube, the Helium emits a pale yellow glow. The glow comes from an electron of the gas becoming excited from the energy. The excited electron leaves its electron shell and orbits the nucleus of the atom at a higher state. Eventually, the electron will return to its original state, releasing the extra energy as light.
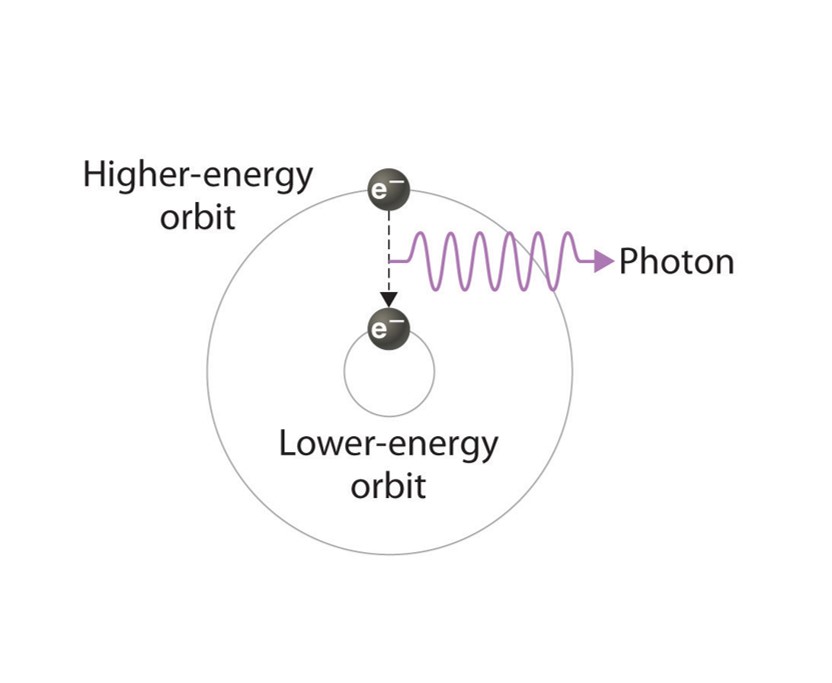 Above: A basic illustration of how an excited electron returns to its regular state
and emits light. Picture courtesy of the open textbook library
Above: A basic illustration of how an excited electron returns to its regular state
and emits light. Picture courtesy of the open textbook library
Back to the Periodic Table
|
|-Onward to the next element!> |
Symbol: He
Atomic Number: 2
Atomic Mass: 4.002602 u
Electron Configuration: 1s2
Year Discovered: 1868
Discovered By: Pierre Janssen, Norman Lockyer


