Neon

Has the era of neon lights ended?
"Neon" dance teams are a relatively new trend. While it only appears as if they're using neon tubing, the performances are mesmerizing!
Contributor: University of Toledo Instrumentation Center
About the Display: The display box features a discharge tube in the shape of the element's symbol. The tube is filled with Neon and powered by a transformer. When an electric charge excites the Neon gas in the tube, the Neon emits a red glow.
Electrons of the neon atoms becoming excited from the energy. The excited electron leaves its electron shell and orbits the nucleus of the atom at a higher state. Eventually, the electron will return to its original state, releasing the extra energy as light.
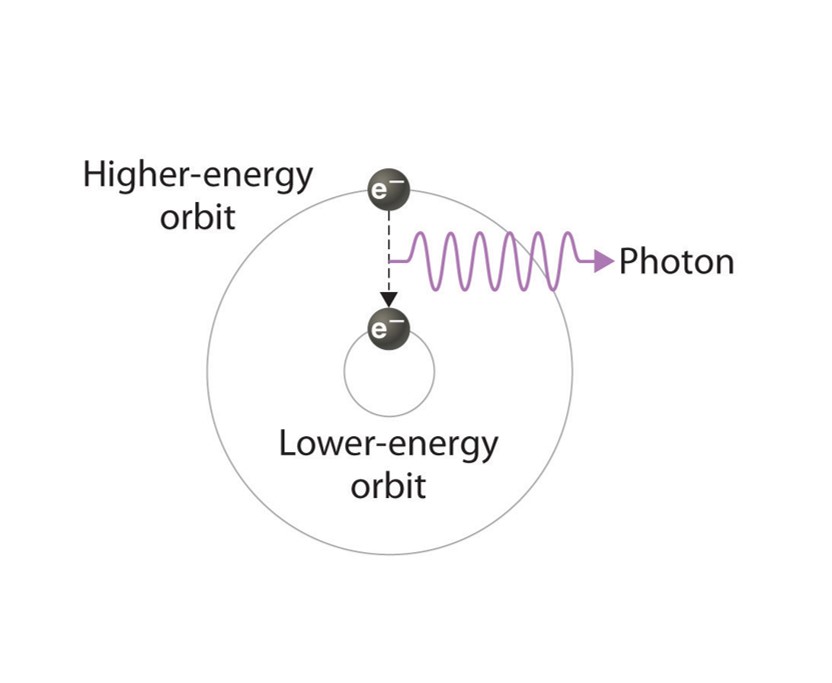
Above: A basic illustration of how an excited electron returns to its regular state and emits light. Picture courtesy of the open textbook library
Back to the Periodic Table
Symbol: Ne
Atomic Number: 10
Atomic Mass: 20.1797 u
Electron Configuration: [He] 2s22p6
Year Discovered: 1898
Discovered By: Morris Travers, William Ramsay


