Vascular Biology Laboratory
Post doc Position Available Research Tech Position Available
our research
Cardiovascular disease remains the number one cause of death in the United States and worldwide. Our research aims to better understand how vascular cells respond and contribute to various cardiovascular diseases, such as hypertension and atherosclerosis. Our objective is to provide novel insights into the mechanisms regulating vascular wall diseases and to identify novel therapeutic strategies for their treatment and prevention.
The vascular wall of our blood vessels is comprised of multiple distinct cell types including vascular smooth muscle cells (VSMCs) and endothelial cells (ECs). Remarkably, VSMCs, which constitute the bulk of the vascular wall, can alter their phenotype in response to multiple vascular wall diseases, such as occlusive vascular wall diseases and systemic hypertension.
Occlusive vascular wall diseases, such as atherosclerosis and post-angioplasty restenosis, are largely dependent on VSMC phenotypic modulation to a proliferative and migratory phenotype that largely contributes to a progressive thickening of the blood vessel wall and narrowing of the blood vessel lumen, which can ultimately lead to life-threatening events, such as myocardial infarction or stroke.
Conversely, systemic hypertension is characterized by increased total peripheral resistance that is attributed to vascular smooth muscle and endothelial dysfunction that ultimately lead to hyperresponsiveness of the blood vessels to local and circulating vasoconstrictors, diminished responsiveness to vasodilators, and to vascular structural changes that culminate in the progressive increase in systemic blood pressure and may ultimately lead to life-threatening end-organ damage, such as heart or kidney failure.
However, the specific genes, signaling networks, and underlying mechanisms that control vascular smooth muscle and endothelial phenotypic modulation in the context of these distinct vascular wall diseases are not fully understood. Delineating these underlying mechanisms will not only lead us to better understand the etiology of occlusive vascular diseases and hypertension but also develop new therapeutic agents for their treatment and prevention.
A) Novel players in Injury-Induced Neointima Formation
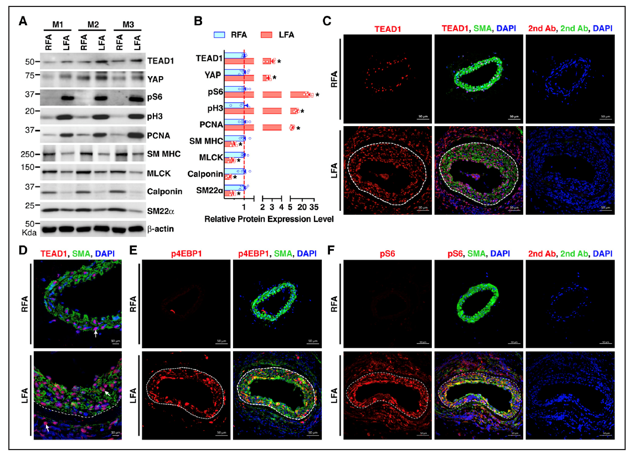
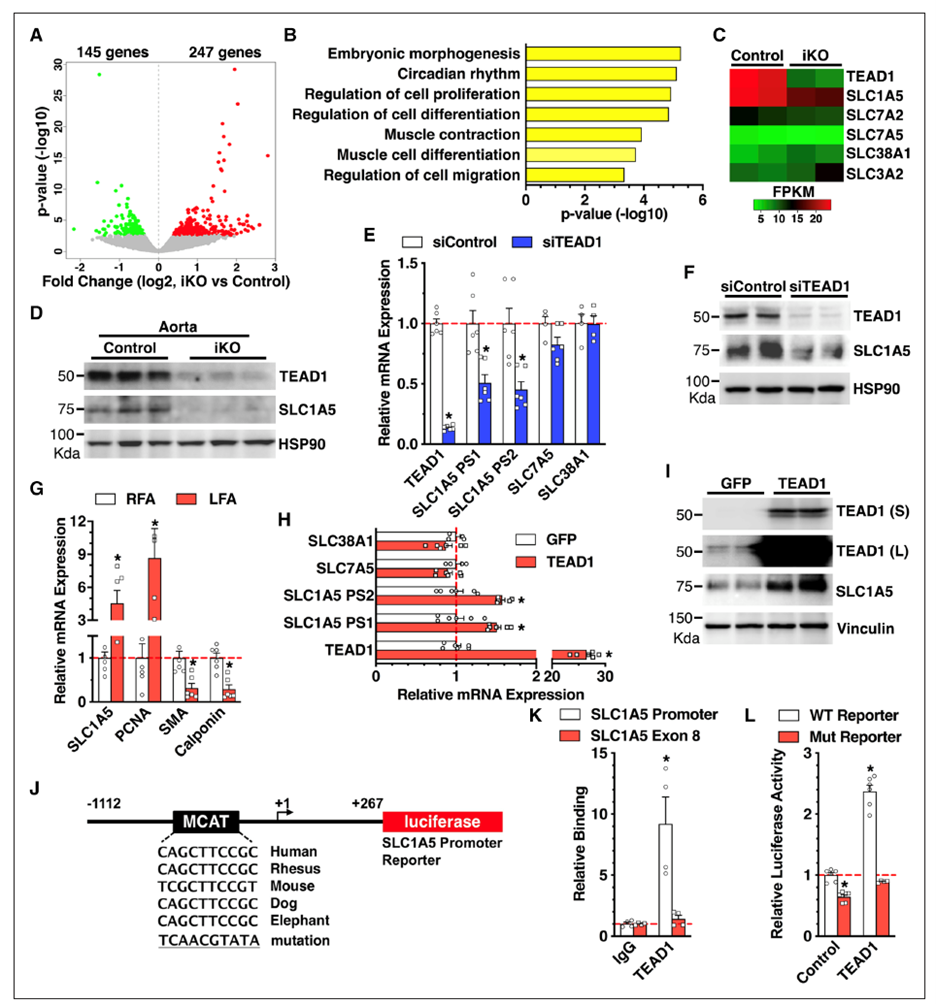
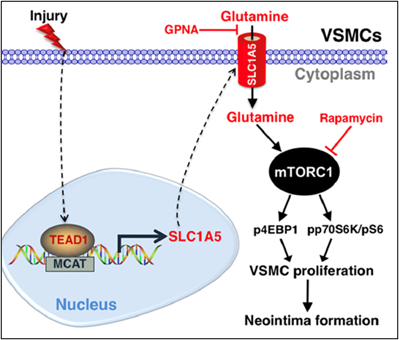
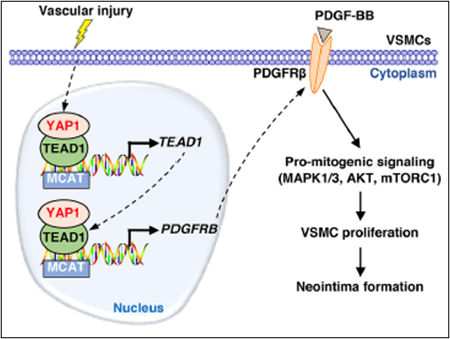
Using smooth muscle-specific tamoxifen-inducible knockout mouse models, we recently delineated the role of two related transcription factors, namely TEAD1 and YAP1, in driving neointima formation (a type of vascular remodeling) in response to vascular injury. Ongoing studies utilize similar approaches to investigate the role of novel genes in injury-induced neointima formation, vascular dysfunction, and hypertension.
Selected Publications:
Osman, I., et al. (2019). "TEAD1 (TEA Domain Transcription Factor 1) Promotes Smooth Muscle Cell Proliferation Through Upregulating SLC1A5 (Solute Carrier Family 1 Member 5)-Mediated Glutamine Uptake." Circ Res 124(9): 1309-1322
Osman, I., et al. (2021). "YAP1/TEAD1 upregulate platelet-derived growth factor receptor beta to promote vascular smooth muscle cell proliferation and neointima formation." J Mol Cell Cardiol 156: 20-32.
![]()
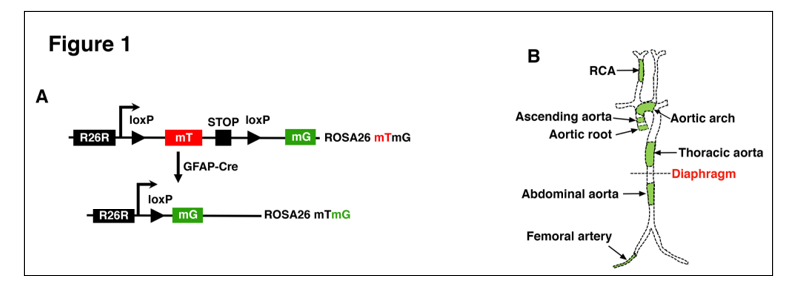
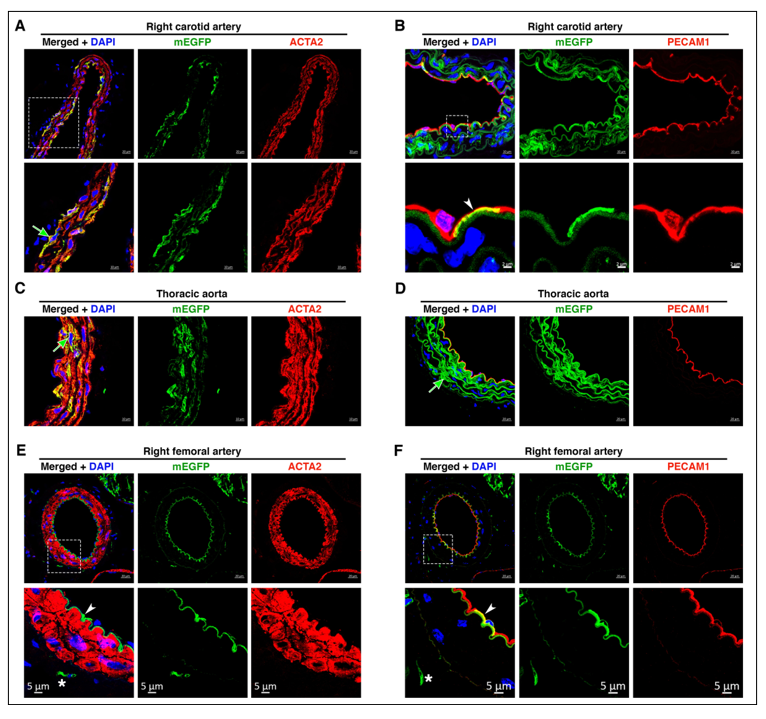
B) The role of GFAP (Glial Fibrillary Acidic Protein)-positive Progenitor Cells in Vascular Development
Using a genetic fate-mapping approach, we recently found that GFAP-positive progenitor cells contribute to the development of VSMCs and endothelial cells in a wide range of vascular beds that have distinct developmental origins. In particular, our findings demonstrate that VSMCs and ECs within the same vascular bed are heterogeneous, where varying proportions of VSMCs and ECs are contributed by GFAP-positive progenitor cells. Ongoing studies focus on the role of GFAP-positive- derived vascular cells in distinct vascular wall diseases.
Selected Publication:
Osman, I., et al. (2020). "GFAP (Glial Fibrillary Acidic Protein)-Positive Progenitor Cells Contribute to the Development of Vascular Smooth Muscle Cells and Endothelial Cells-Brief Report." Arterioscler Thromb Vasc Biol 40(5): 1231-1238