Frequently Asked Questions About Animal Research Protocols
The questions listed below are a snapshot of those that frequently come up regarding
animal research protocols.
Please contact the IACUC Administrator (IACUC@UToledo.edu) if your question or concern is not addressed below.
Updated: February 3, 2022
- What types of animals require an IACUC protocol?
All vertebrate animals (animals with a backbone) require an IACUC protocol. Higher invertebrates also require an IACUC protocol and include: octopus, squid, nautilus, lobsters, crabs and hermit crabs, and crayfish. - What type of protocols can I submit?
Protocols can be submitted as combination research and breeding (preferred), research only, breeding only, or instruction. If you will be breeding animals for a specific research protocol, the IACUC prefers that you submit one combined research/breeding protocol.
- What is the submission deadline for my IACUC protocol?
- 12th of the Month = Deadline for Submission for Pre-Review
- Last Business Day of the Month = Deadline for Submission for Upcoming Meeting
If you want the best chance at approval, you should submit your protocol through IRB Manager as soon as possible in order to obtain a pre-review by the IACUC Administrator and the Attending Veterinarian.
-
How do I submit a protocol?
Please see the IACUC IRB Manager Training website for instructions on how to log in and submit a protocol. - How long is protocol approval good for?
If a protocol involves the use of a USDA-covered species, approval is good for one year and the active protocol must be reviewed annually. Regardless of species, all protocols must be renewed ever three years. This means that a new protocol must be filled out and submitted to the IACUC every three years for all active protocols. - My grant is for five years but the protocol says that I can only get approval for
three years. How do I get an extension?
The three-year limit on animal protocols is explicitly stated in the Federal regulations. Extensions cannot be given for any reason. - What are some examples of experimental timelines?
Experimental timelines need to include all experimental procedures and can be text such as: ‘time 0=begin experimental feeding, weeks 1/3/5=blood draw, week 10=euthanize animals’. Experimental timelines can also be figures such as:
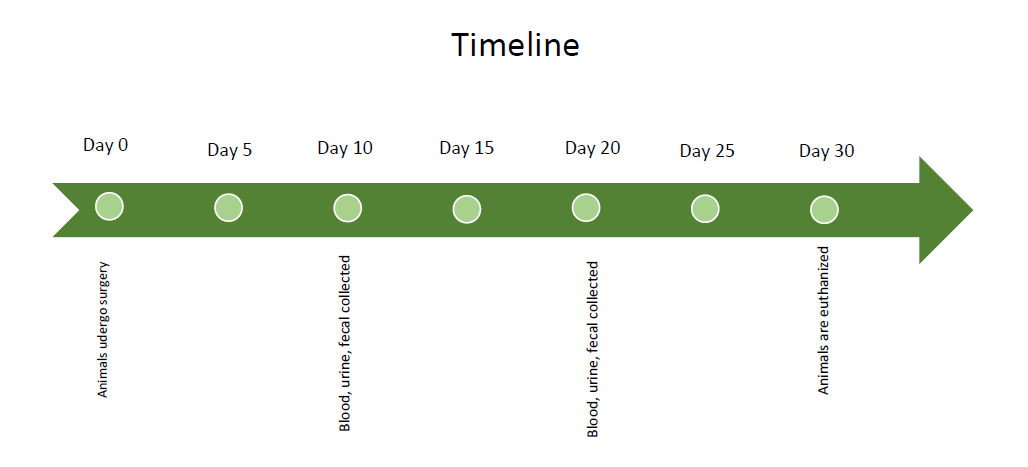
- Should I amend my protocol or apply for a new protocol?
Amendments to an approved protocol are appropriate if the changes do not significantly alter the main objective/scope of the protocol. Examples of appropriate amendments includes: change in principle investigator, personnel, number or type of procedures, number of animals, type(s) of euthanasia, types of anesthesia, etc. - When does field work with animals require an animal research (IACUC) protocol?
Any study that disrupts, interferes, or otherwise disturbs the natural behavior or patterns of animal requires an IACUC protocol. Example, you place bird feeders inside a wooded area and outside a wooded area in order to observe differences in feeding. The placement of bird feeders disrupts the birds natural feeding behavior. This study would require an IACUC protocol - Does taking tissues from dead animals require an animal research (IACUC) protocol?
An IACUC protocol is required if animals are to be euthanized in order to obtain tissues or samples. If the animals are already deceased (i.e., tissues from a slaughterhouse, euthanized as part of another IACUC protocol), then you do not need an IACUC protocol to obtain post-mortem samples. Institutional Biosafety Committee (IBC) approval may be necessary, however. - What do I do if I have a complaint or concern about animal care or animal welfare?
The IACUC takes all concerns regarding the care and use of animals at the institution seriously, regardless of who submits the concern. Concerns or complaints may be submitted via email or phone and may be submitted anonymously. Contact IACUC Administrator, IACUC Chair, or Attending Veterinarian. - Can I use drugs purchased from Sigma® in research with animals?
IACUC regulations require that pharmaceutical grade drugs must be used in animal research. Chemical companies, such as Sigma®, do not supply pharmaceutical grade drugs. To ensure that pharmaceutical grade drugs are being used, they must be purchased from reputable drug companies such as:
Alternatively, non-pharmaceutical grade drugs may be used in animal research with scientific justification within the protocol and approval by the IACUC. Examples of such justification may include, but are not limited to:
- No equivalent veterinary or human drug is available for experimental use.
- Although an equivalent veterinary or human drug is available, dilution or change in
formulation is required.
- Although an equivalent veterinary or human drug is available for experimental use,
the analytical or chemical grade reagent may be required to replicate methods from
previous studies if it is the only option to produce results that are directly comparable.
- The available human or veterinary drug is not concentrated enough to meet experimental requirements.



