Ruili Xie, Ph.D.

Assistant Professor
Office: 186 Block Health Sciences Building
Tel: 419-383-6439
Lab: 419-383-4201 (HSB 108)
Fax: 419-383-3008
Email: ruili.xie@utoledo.edu
Education:
B.S. 1997 Peking University, Beijing, China
M.S. 2000 Institute of Genetics, Chinese Academy of Sciences, Beijing, China
Ph.D. 2006 University of Texas at Austin, Austin, TX
Research Interest:
Hearing loss becomes prevalent more than ever due to the steady increase of our life
expectancy (leads to the increase in age-related hearing loss, or presbycusis), and
over-exposure to sound from sources like personal electronic devices (leads to noise-induced
hearing loss). I study how the auditory nervous system processes sound, and how the
neural processing is disrupted under aging and hearing loss conditions. The primary
goals of my research are to elucidate the mechanisms at the molecular, cellular and
circuit levels that underlie normal hearing as well as hearing loss conditions due
to aging and noise trauma.
Research Techniques:
Our studies primarily utilize whole-cell patch clamp recording technique to investigate
the synaptic transmission in mouse brain slices. We also combine other experimental
approaches including pharmacology, immunohistology, molecular biology, optogenetics,
and behavioral techniques in our research.
Research Summary:

The animal model of my research
Like people, mouse loses hearing during aging and after noise trauma. They share
the same mechanisms of auditory nervous system with us, yet are small and stable enough
for breeding and experimental manipulations. Various types of transgenic and mutant
mice are readily available and more are being developed for the neuroscience research.
They are ideal to use in studying aging and hearing loss. Picture shows three young
mice at 1 month and an old one at 19 month.
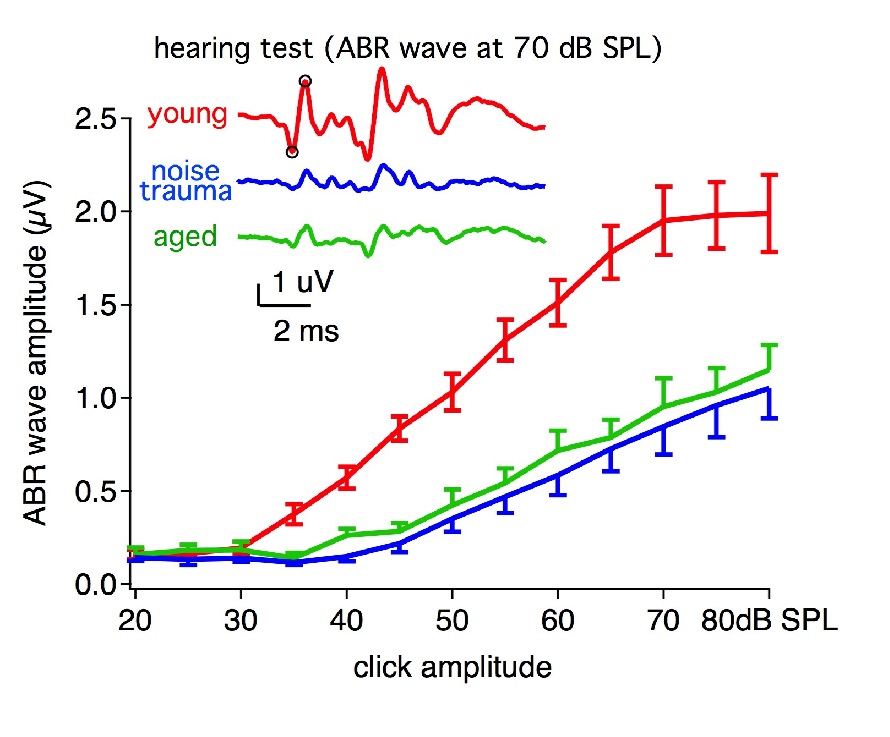
Hearing test – (ABRs)
We assess mouse hearing by recording sound evoked auditory brainstem responses (ABRs).
Hearing loss is seen in aged mice(19 month) and mice with noise trauma, in terms of
ABR waveform amplitude. Inset: example ABR waveform to a 70 dB SPL click. Red: young
mouse (3 month); Green: aged mouse (19 month); blue: young mice with noise trauma
(3 month).
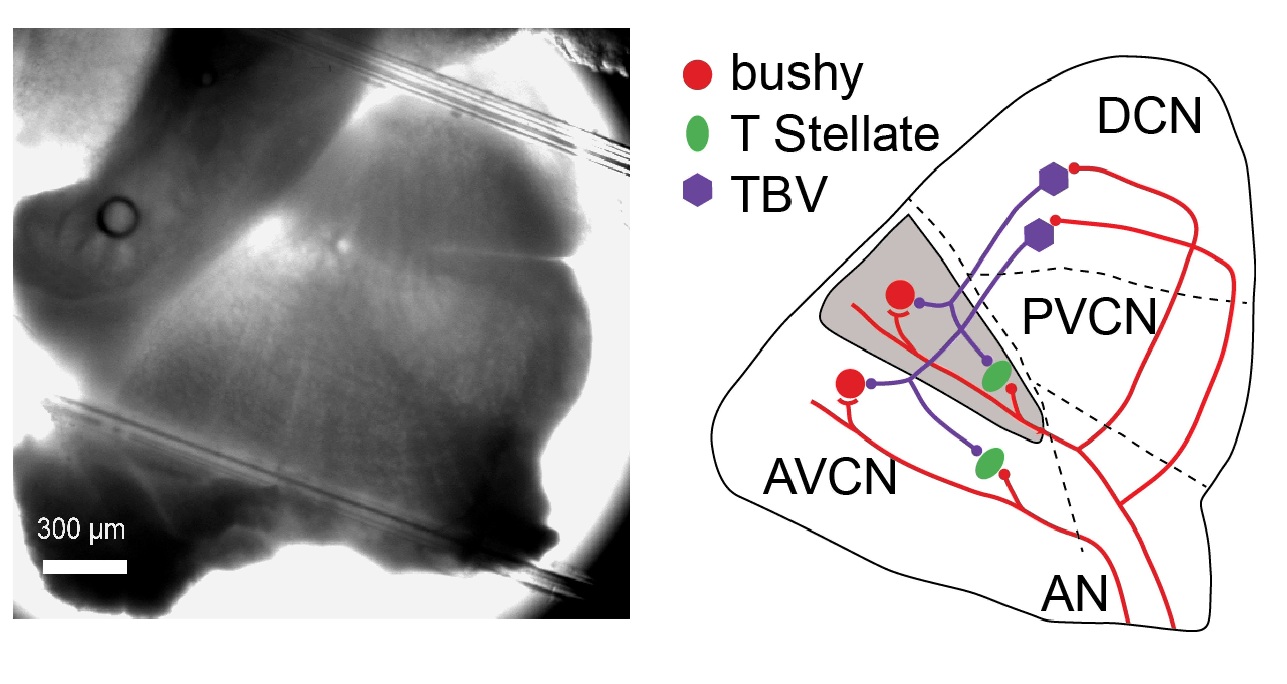
The cochlear nucleus (CN)
An image of the cochlear nucleus from a mouse brain slice (left), and the diagram
of a simplified CN neural circuit (right). Different principal cell types in the
CN process distinct aspects of the sound information. For example, bushy cells encode
the fine temporal information of the sound structure, which is critical for sound
localization as well as the performance of complex auditory tasks like speech recognition.
The neural encoding of such fine temporal information is deteriorated during aging
and hearing loss, which makes the bushy cells and the synapses they receive good targets
of research. Red lines: excitatory auditory nerve (AN) projections. TBV: tuberculoventral
cells that project glycinergic inputs to both bushy and T-stellate cells. Three regions
of the CN are AVCN (anteroventral CN), PVCN (posteroventral CN) and DCN (dorsal CN).
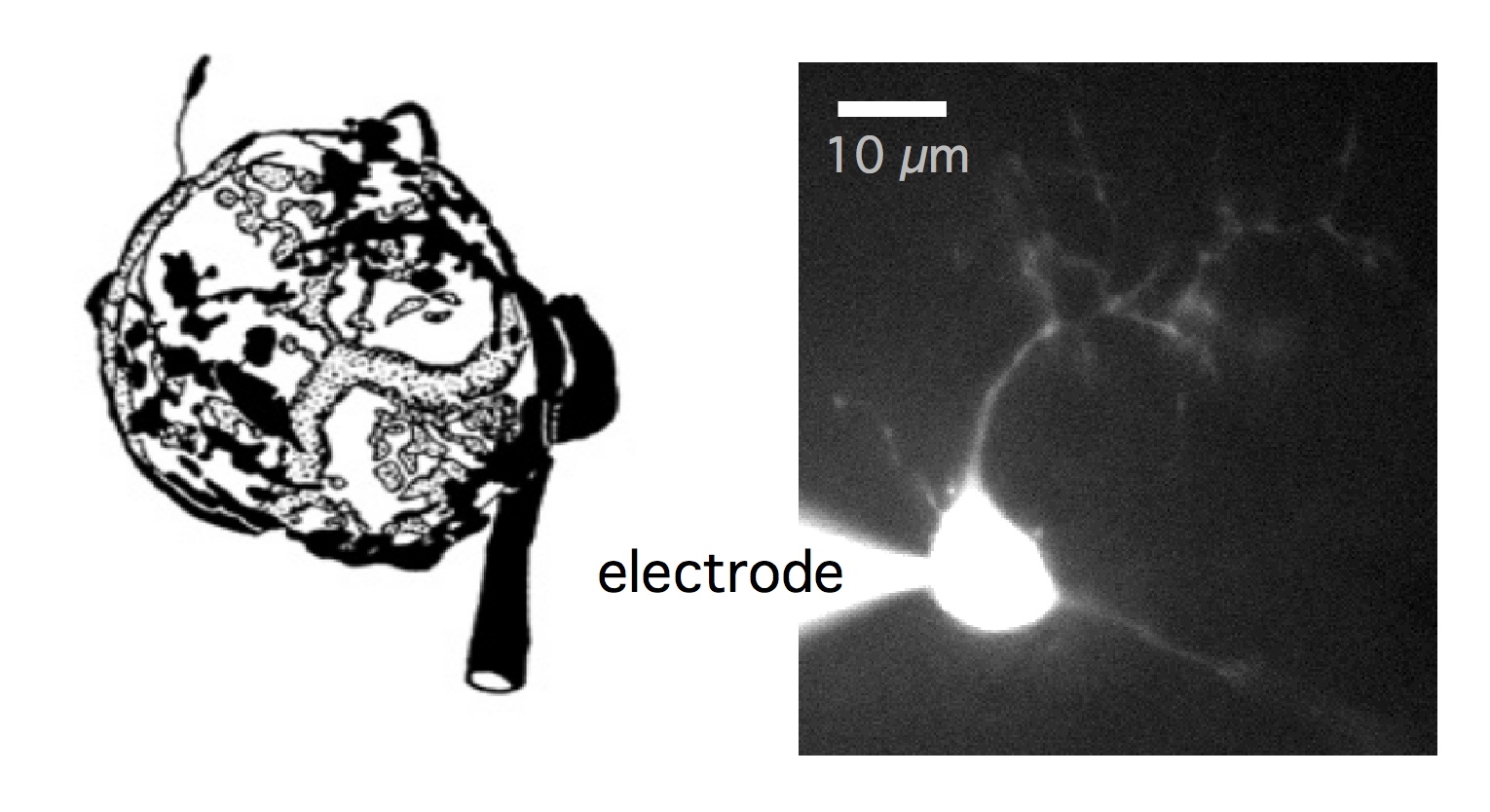
Endbulb of Held synapses and bushy cells
Left: drawing of the endbulb of Held (by O’Neil et al, 2011) to show the giant synapse
that wraps around the soma of the postsynaptic bushy cell. The synapse is specialized
to faithfully transmit sound information with temporal precision. Right: an example
bushy cell patched during whole-cell recording. Cell was filled with Alexa Fluor 488
for visualization.
Synaptic transmission is compromised during aging and hearing loss
Voltage-clamp recordings of EPSCs in bushy cells to stimulations of the endbulb of
Held synapse. Notice that the synchronous EPSC responses were large and well-timed
to the stimulus onset (tick marks on top), but were small and less well-timed in aged
mice and mice with noise trauma. The asynchronous (or delayed) EPSC responses were
rare in young mice, but increased a lot in aged and noise trauma mice. Both types
of responses were partially rescued by bathing the slice in EGTA-AM in mice with noise
trauma (Noise Trauma + EGTA), suggesting that presynaptic calcium levels are elevated
under aging and hearing loss conditions. Only the last 5 responses of the 50 pulse-train
at 400 Hz were shown.
Optogenetic tools for the study of inhibitory mechanisms
Top: 2-photon image of the cochlear nucleus from a VGAT-EYFP-Channelrhodopsin-2 mouse,
which express EYFP-ChR2 fusion protein in inhibitory neurons (VGAT positive neurons).
Red arrow: an inhibitory neuron with EYFP fluorescence under 2-photon excitation
(975 nm wavelength). Green arrows: shadow of non-EYFP-expressing cells (excitatory
neurons, including bushy cells). Bottom: light stimulation (2-photon at 940 nm wavelength
to excite ChR2) evoked spikes in ChR2 expressing inhibitory neurons, and evoked IPSPs
in bushy cells that receive inputs from inhibitory neurons.
Research Grants:
NIDCD R01 grant, #R01DC016037: Cellular mechanisms of age related hearing loss (2017-2022,
active). Principal investigator: Ruili Xie. Total award: $1,881,315 (Direct: $1,250,000;
Indirect: $631,315).
NIDCD small research grant (R03), #R03-DC013396: Synaptic mechanisms underlying noise-induced and age-related hearing loss (2013-2017, completed). Principal investigator: Ruili Xie. Total award: $456,000 (Direct: $300,000; Indirect: $156,000).
References:
1. Xie R, Manis PB. Intrinsic excitability and synaptic properties of auditory nerve input
of radiate and planar multipolar neurons in the mouse anteroventral cochlear nucleus.
Under review.
Book Chapter:
Manis PB, Xie R, Wang Y, Marrs GS, and Spirou GA (2012). The Endbulb of Held. In: Springer Handbook of Auditory Research (Vol. 41): Synaptic Mechanisms in the Auditory
System. pp 61-93. Edited by Trussell LO, Popper AN and Fay RR. New York: Springer.
For latest publications, please check my bibliography URL:
NCBI


