Advanced microscopy & imaging center
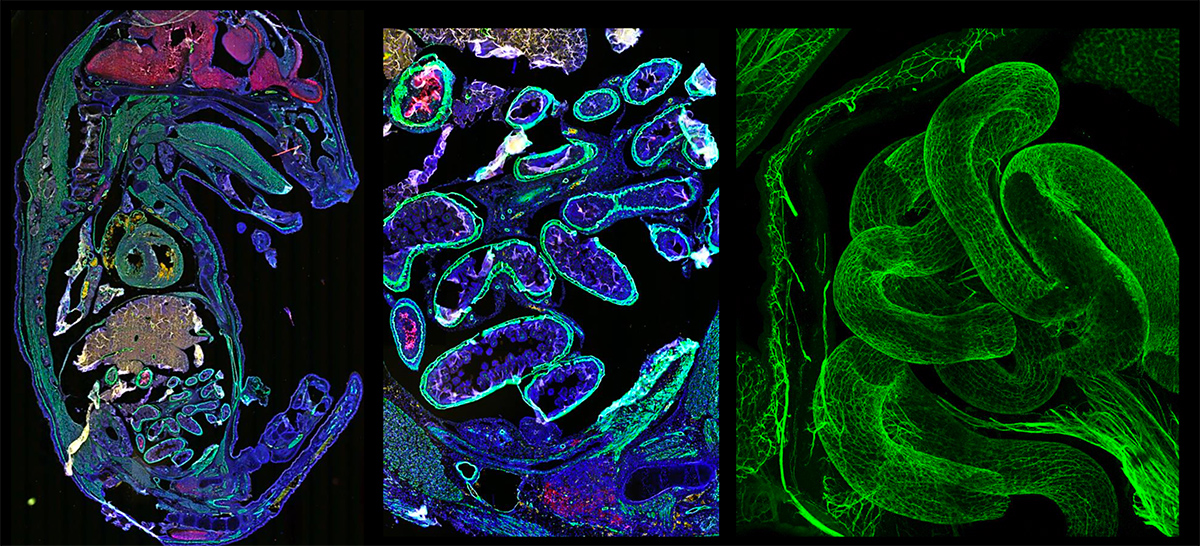
The Advanced Microscopy & Imaging Center (AMIC) on the Health Science Campus is a 3,000-square-foot facility designed to bring together advanced light and fluorescence microscopy systems and “state of the art” image analysis software to perform biomedical research. Current projects in this center include work in Cancer, Genetics, Neurobiology, Vascular Biology, Pathology, Pulmonology, and Arthritis research. The Center consists of a 1,000 square foot General Microscopy Laboratory with 12 separate “work stations” housing individual, computer-based microscopy systems. The center also includes an 800-square-foot, Tissue-culture Microscopy Laboratory with 6 separate “work stations” for microscopy systems dedicated to the study of living cells.
The Advanced Microscopy & Imaging Center is diversifying the ease and productivity of research that can be performed at the University of Toledo by including: live cell imaging and kinetic analysis (IncuCyte S3), analysis of cellular and mitochondrial metabolism (Seahorse XFe/XF96), user-friendly automated imagers to produce perfect western blot images (Syngene Western Blot Imager), and numerous other instruments to aid in cellular analytics. Once properly trained most of these instruments are fee for service and are available as shared equipment for all interested parties.
The AMIC is located in the basement of the Block Health Science Building room 057. For after hours access please fill out this form.
For more information on each of the instruments in the AMIC simply click on the image.
For questions or to inquire about the AMIC please contact Dr. Andrea Kalinoski via email or 419.383.4205
amic Instruments
For more information simply click on the image.
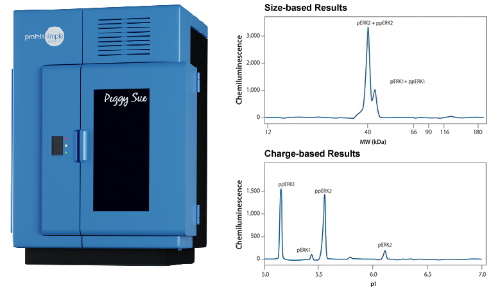
The Peggy Sue™ Protein Analyzer
The Peggy Sue™ (Protein Simple) lets you separate and analyze proteins by size or charge from 2-440 kDa either by immunoassay or total protein. Small sample volumes and starting materials are no problem. This instrument can use as little as 0.2 µg/µL of protein in just 5 µL of sample, and runs up to 96 samples in one experiment. It can record up to 96 data points overnight and contains software for all needed data.
Seahorse XFe Analyzers
Two Seahorse XFe Analyzers (Agilent) an 8-well and 96-well format are available to measure the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of live cells in a multi-well plate to interrogate key cellular functions such as mitochondrial respiration and glycolysis. The instruments perform compound addition and mixing, label-free detection, and automatic calculation of OCR and ECAR in real time. Click here for the Cell Reference Database
TIRF (Total Internal Reflection Fluorescence) Microscope
The Olympus TIRF Microscope is available on an Olympus IX81 inverted microscope/imaging system that allows the visualization of fluorescent molecules either in wide-field (conventional) or exclusively at the cell-glass interface (TIRF). This latter capability allows selective, real-time tracking of single molecule or particle dynamics at the surface of living cells with three solid state laser lines (488, 543 and 633).
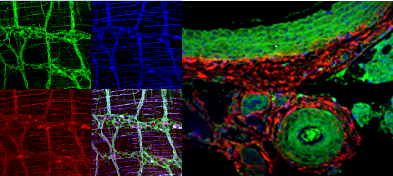
leica tCS sp5 multiphoton laser scanning confocal microscope
SP5 Laser Scanning Confocal Microscope with MP (Leica Microsystems) is equipped with both conventional and high-speed resonance scanners. Including conventional lasers plus multi-photon excitation (458, 488, 514, 561, 633, and a tunable Ti-Sapphire MP laser 710-990nm). It is capable of collecting up to five colors simultaneously for quantitative confocal image analysis in both live cell & animal imaging, fixed tissue and includes the capabilities for 3-D reconstruction, FRAP, FRET, animation, stereo imaging, single layer projection, time lapse collection, and co-localization analysis.
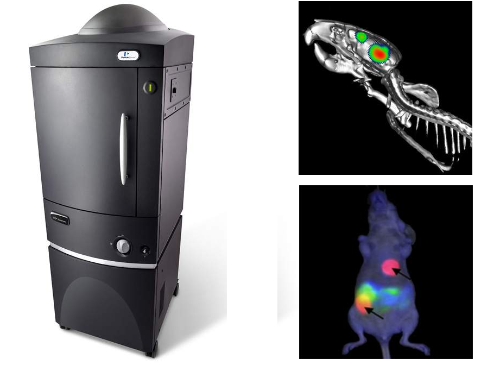
the ivis spectrum
The IVIS Spectrum (Caliper Life Sciences) is a whole animal fluorescence imaging system. The IVIS Spectrum is a multimodal bioluminescent and fluorescent imaging system specifically designed for noninvasive longitudinal imaging of cells and tissues in small animals. This instrument facilitates the study of biological processes via fluorescence in small animals, including tumor growth, cancer metastasis, bacterial infections, immune responses and inflammation, and regulation of tissue-specific gene expression. Overview PDF.
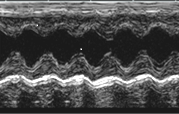
Acuson Sequoia C512 Ultrasound
The ACUSON Sequoia C512 cardiac Ultrasound (Siemens) imaging system is an echocardiography system used for studying cardio-vascular disease processes in small animals, including ischemic heart disease, heart failure, hypertension, diabetic cardiomyopathy, cardiac hypertrophy and remodeling. Capabilities include: high-resolution imaging, tissue harmonic imaging, differential echo amplification, spectral Doppler (pulsed and continuous wave), color Doppler (for measurements of velocity energy and tissue Doppler imaging) and color-Doppler M-mode imaging.
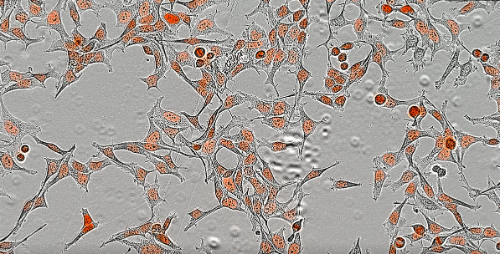
IncuCyte S3 Live-cell Analysis System
The IncuCyte S3 Live-cell System (Sartorius) is a real-time system that sits inside a standard tissue culture incubator and automatically acquires and analyzes HD, phase and fluorescent images of living cells, around the clock, for days, weeks, or months, while cells remain undisturbed. Kinetic, image-based measurements ensure you never miss a relevant response allowing for cell monitoring and surveillance, cell health and viability, migration and invasion, plus a wide range of phenotypic cell-based assays.

G:Box Western Blot Imagers
Two G:Box Imagers (Syngene) are available for researchers. Each of these systems include: a top of the range, cooled cameras with RGB & IR lighting options. The imagers are set up to image fluorescent DNA and RNA gels, protein gels stained with visible dyes such as Coomassie blue, chemiluminescent and multiplex fluorescent Western blots. The imager found in the Health Education Bldg. rm 233A is capable of IR, RGB, Chemi, and UV detection while the imager located in Block Health Science Bldg. rm 057, is only capable of chemi & UV detection.
The Cytation™ 5
The Cytation™ 5 (BioTek) is a cell imaging and plate reader system that combines automated digital widefield microscopy with conventional multi-mode microplate reading. With 4X, 20X and 40X magnification, the imaging module provides high-quality cellular and sub-cellular imaging in fluorescence, brightfield, color brightfield and phase contrast. The multi-mode microplate reader incorporates variable bandwidth monochromator optics and high sensitivity filter based detection optics. Live cell imaging and multi-mode assays are optimized with incubation to 65 °C with shaking, and dual reagent injectors with Gen5 software. ***Found in the Health Education Building, room 233A.
Leica Laser Microdissection
LMD, also known as Laser Capture Microdissection or LCM, enables users to isolate specific single cells or entire areas of tissue. Powered by a unique laser design and dynamic software, Leica LMD systems allow users to easily isolate Regions of Interest (ROI) from entire areas of interest.
AID EliSpot Reader
AID EliSpot plate reader for fast and efficient EliSpot interpretation. The AID interprets any type of 96- and 384-well plates, including all brands of membrane type plates, ELISA-style plates and low volume plates. It simultaneously takes high resolution images, auto-centers the well, counts/analyzes plates and exports data in various formats as needed.


