Titanium

Contributor: Olivia Schaffner’s AP Chemistry class, Edgerton High School; Laurel and Suzana Leber (Titanium dental implant)
About the Display: This display currently features - there is more to come:
- Dental Implant made of titanium donated by Laurel and Suzana Leber
- Piece of glass coated with Titanium Oxide* donated by Dean Giolando
- Wedding ring made of titanium
- Toothpaste: contains titanium dioxide (TiO2), which is an intensely white pigment with good luster and endurance. This is what gives toothpaste its white color.
- Sunscreen: Titanium dioxide, which is described above, is used in many sunscreens because it prevents UV rays from reaching the skin by reflecting them, similar to how white pigment reflects light.
- Drill bits: Titanium drill bits are not actually solid titanium; rather, they consist of a coating of titanium nitride applied to a high speed steel (HSS) drill bit. The titanium nitride gives them an incredibly high surface hardness and makes them very corrosion-resistant. These drill bits are ideal for cutting through metal and last up to six times longer than standard HSS drill bits.(currently not in box)
- Paint: Titanium dioxide is a white pigment, described above, and is the main pigment used in white paint.(currently not in box)
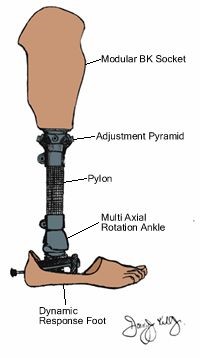
- Prosthetic leg pyramid attachment: Prosthetics are often made of titanium or an alloy that is 90% titanium because it
is very strong, highly corrosion-resistant, and close to the same density as bone.
This particular piece is a male pyramid attachment that attaches a socket to a prosthetic
knee or pylon, which can be seen labeled “Adjustment Pyramid” in the image to the
right. (currently not in box)
- Femoral component of replacement hip: Replacement joints are often made of titanium for the same reasons listed above for prosthetics. In addition, titanium does not react with substances in the body, making it an ideal material to be placed inside the body, and it has a high bonding ability with bone. This particular piece is the femoral component of a replacement hip that was placed in Rusty Schaffner (father of Olivia Schaffner, the teacher of the contributing class) and has since been removed. Rusty was in a car accident in 1996 that completely crushed the left side of his pelvis, and he has since had four hip replacements. This piece was the first of the four, and this operation was performed by none other than a University of Toledo Medical Center orthopedic surgeon. In a hip replacement, the stem of the femoral component is inserted into the femur, and the head rests in the acetabular component (the socket), which is attached to the pelvis. This can be seen in the image below.
- Golf club head: Many golf clubs are made of titanium because it is lighter, stronger, more elastic, and more corrosion-resistant than other metals. Golf clubs need to be swung fast and need to bend a little through the swing, so titanium matches these requirements very closely.
- Chewing gum: Chewing gum contains titanium dioxide, which is used as a coloring agent to make the gum white.
- Model submarine: Titanium is used in the hulls of submarines and ships because it makes them lighter and much less likely to corrode than if they were made of steel.
- Model aircraft: Titanium is used in the engine, frame, and landing gear of aircraft and spacecraft because it is lightweight and has a high temperature resistance.
- Model bicycle: Titanium is used to make lightweight bicycles because it is strong, durable, less dense than steel, and more corrosion-resistant than steel.
- Tooth implant/Fake teeth: Tooth implants are made with titanium or a titanium alloy for the same reasons that replacement joints are made with titanium; it is highly biocompatible and has a high bonding ability with bone.
- Broadhead: Hunting arrowheads are often made of titanium because it is very strong, making it durable and allowing it to easily pierce whatever it needs to. It is also very lightweight, allowing the arrow to travel quickly while not affecting the balance of the arrow or how it falls during flight.
- Rock: Titanium metal and titanium dioxide are obtained from ores that are found in rocks in the earth’s crust.
Fun Facts:
- Titanium was named after the Titans of Greek mythology.
- Titanium is as strong as steel but is half as dense.
- Titanium is the ninth most abundant metal in Earth’s crust.
- Titanium has 18 isotopes, but only 5 of them are stable.
- Titanium is so resistant to corrosion that containers are made out of it to store nuclear waste.
- Even though titanium was discovered in 1791, it was not named until 1795.
- Titanium is the only element that is able to burn in nitrogen.
* The panel of glass in the display is self-cleaning and was developed here in Toledo! For more information, visit https://www.pilkington.com/en/us/products/product-categories/self-cleaning/pilkington-activ-range/pilkington-activ-clear!
About the Contributor: Ms. Schaffner’s AP Chemistry class from Edgerton High School in Edgerton, Ohio, has 13 juniors and seniors in it. Pictured in the front row are Leeanna Pelz, Claire Flegal, Kendra Blue, Madissen Fritch, Hanna Hug, and Rebecca Schroeder. Pictured in the back row are Devin Thiel, Micah Ritter, Braydon Cape, Eric Herman, Brody Degryse, Greg Roth, and Milan Thomas.
Laurel and Suzana Leber visited the UT Chemistry Department this past summer as a
future Rocket and a future Rocket mom!
Back to the Periodic Table
Symbol: Ti
Atomic Number: 22
Atomic Mass: 47.867 u
Electron Configuration: [Ar]4s23d2
Year Discovered: 1791
Discovered By: William Gregor



