Immune Tolerance laboratory
Humanized mouse model for spontaneous type 1 diabetes
Although the NOD mouse is the current standard for studies of type I diabetes (T1D), no curative therapies resulting from this model have passed clinical trials. Knock-in/out models cannot be used to mimic the total pathophysiology of diabetes. In order to develop a cure for T1D, there is a need for an animal model of human T1D that naturally resembles the human disease and as such exhibits the complications of diabetes. In the model herein, mouse MHC-II molecules were replaced with human DQ8 in all antigen presenting cells. Our mice also express human Glutamic Acid Decarboxylase 65 isoform (GAD65) in pancreatic beta cells. Importantly, our mouse model has a genetic milieu that seems to compromise beta-cell neogenesis and/or regeneration that makes it similar to humans. Our humanized mouse model has the closest known phenotype to human T1D and is most suitable for addressing pathophysiology and treatment of diabetes and its complications.
Diabetes-Associated Dry Eye Syndrome in a New Humanized Transgenic Model of Type 1 Diabetes
CAR-Treg therapy: We have developed pancreatic beta-cell, antigen-specific, Chimeric Antigen Receptor (CAR) T regulatory cells (Tregs) and explored their therapeutic potential against T1D in human tissues ex-vivo and in a humanized mouse model in-vivo. We show here that human, pancreatic antigen-specific, CAR-Tregs home to human pancreatic islets and suppress diabetogenic cytotoxic T cells ex-vivo. Same CAR Tregs allow for Type 1 Diabetes (T1D) reversal in-vivo in a spontaneous humanized T1D mouse model.
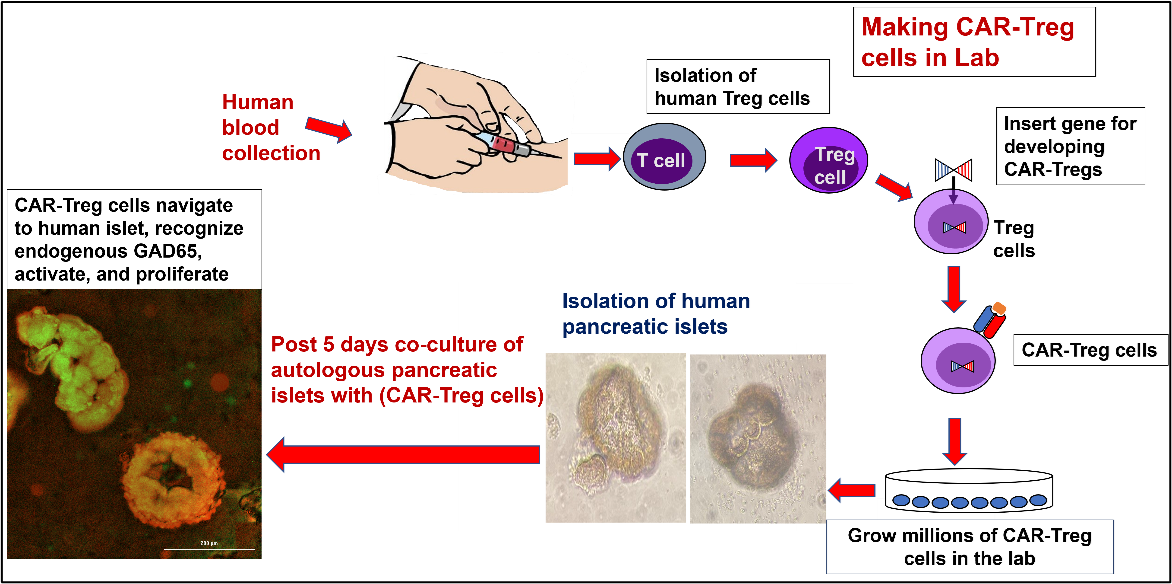
Adoptive Cell Transfer for Type 1 Diabetes Reversal
Homing of Antigen-specific Engineered Regulatory T Cells To Human Pancreatic Islets
Exploiting the Plasticity of Teffector cells: Antigen-specific Teffector (CD4/CD8) cells can be induced (plasticized) to become Treg cells by using low dose immune modulators. T cells can adopt alternative transcriptional lineage programs that generate functionally distinct subsets to modulate localized or specific inflammatory sites. We have identified two key pathways that are able to push Teffector cells towards a Treg phenotype.
Recently, we have shown in vitro simultaneous inhibition of (eIF5A+Notch) signaling using (GC7+anti-DLL4) mediates suppression of diabetogenic T cells by inducing CD4 T cells co-expressing IL-17+IFNg+ (CD4+IL-17+IFNg+) to take on a Treg phenotype. We have shown this simultaneous approach works efficiently in mouse T cells isolated from the PLN (Imam et al., 2021) as well as in human blood T cells from patients with recent onset of latent autoimmune diabetes in adults (LADA).
eIF5A inhibition influences T cell dynamics in the pancreatic microenvironment of the humanized mouse model of Type 1 Diabetes
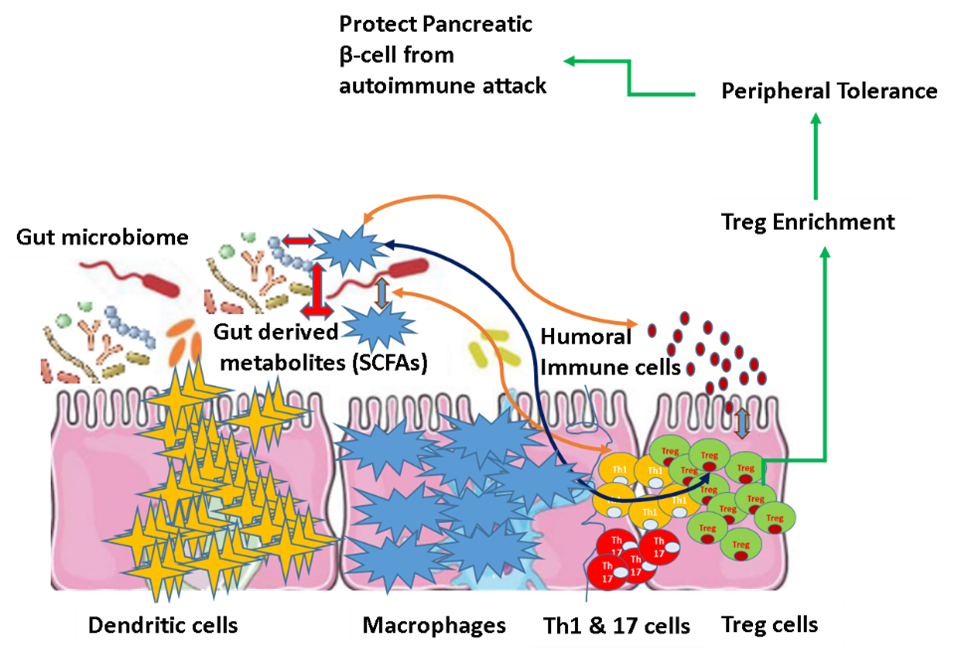
Gut Microbiota and Type 1 Diabetes: Multi-omics modeling of the Gut Microbiome and Host Response in the Progression of Type I Diabetes. During the first three years of human life, the gut microbiota undergoes a dynamic change in taxonomic diversity, and this is the time during which T1D disease progression starts. We are also investigating the dynamic changes of the intestinal microbiome with host pathophysiological signatures in a longitudinal study to develop predictive models associated with the onset and progression of Type I Diabetes (T1D). (Article submitted to BMC Microbiome). The work was awarded with Helmsley Charitable Trust Outstanding Abstract Award in Type 1 Diabetes at ENDO 2019 conference.
The Programmed Death 1 Ligand 1 (PD-L1) in Autoimmune diseases: A newly FDA approved immune checkpoint inhibitor immunotherapy like "Nivolumab" is giving a hope for treating several cancers. Nivolumab works by blocking PD1-PDL1 pathway which is a pathway used by cancer to evade the immune system. Unfortunately, this treatment comes with its own complications, a serious side effect of autoimmune cases like type 1 diabetes has being increasingly reported after the treatment of Nivolumab, These reports are hinting the importance of the PD1-PDL1 pathway in maintaining pancreatic islet self-immune tolerance. In my current project, we are exploring the involvement of PD1-PDL1 pathway in the pathobiology of Type I Diabetes (TID). Specifically, our aim is to elucidate critical genetic mechanisms affecting the PDL1 expression in pancreatic islets of TID patients, and to exploit these mechanisms for developing targeted therapeutics against T1D.
Developing an immuno-genomic marker for early detection of thyroid cancer: Thyroid cancer is predicted to become the third most common cancer in women and the total estimated cost for treatment is around $1.5 B annually (NCI, 2020). Due to lack of accurate tests, the prevalence of thyroid cancer identified during thyroid surgery varied from 10.5% to 55.4% (Kim et al., 2020). We interviewed endocrinologists using NSF Innovation Corps; endocrinologists expressed great concern, and stated that, “Sometimes it is extremely frustrating for patients to undergo surgery despite there being no cancer.” The primary goal of the molecular diagnostic test is to correctly identify benign nodule among those with intermediate cytology, thereby decreasing the number of unnecessary diagnostic surgeries. Currently three main molecular tests available, ThyroSeq v3, ThyGeNEXT and ThyraMIR, and Afirma GSC and Xpression Atlas, have sensitivities of 94%, 93%, 91% and specificities of 82%, 62% and 68% respectively (Nishino et al., 2021). Recently our lab has patented a technology for the diagnosis of thyroid cancer based on frequency of CD3+CD4-CD8- (Double Negative, DN) T cells as a prognostic as well as diagnostic marker (Imam et al., 2021). The hypothesis was validated statistically and thus the patient is at higher risk of cancer if the frequency of DN T cells is >9.14%, the sensitivity of test being 96.6% and specificity 100%. The high specificity of the test is promising since it abolishes the false positive diagnosis and unnecessary surgical procedures.
Application:
- This is the first biomarker for thyroid cancer diagnosis based on cellular crosstalk.
- This present finding identified a diagnostic cell profile to reduce the ⁓70% unnecessary thyroid surgeries where FNA cytology results are inconclusive.
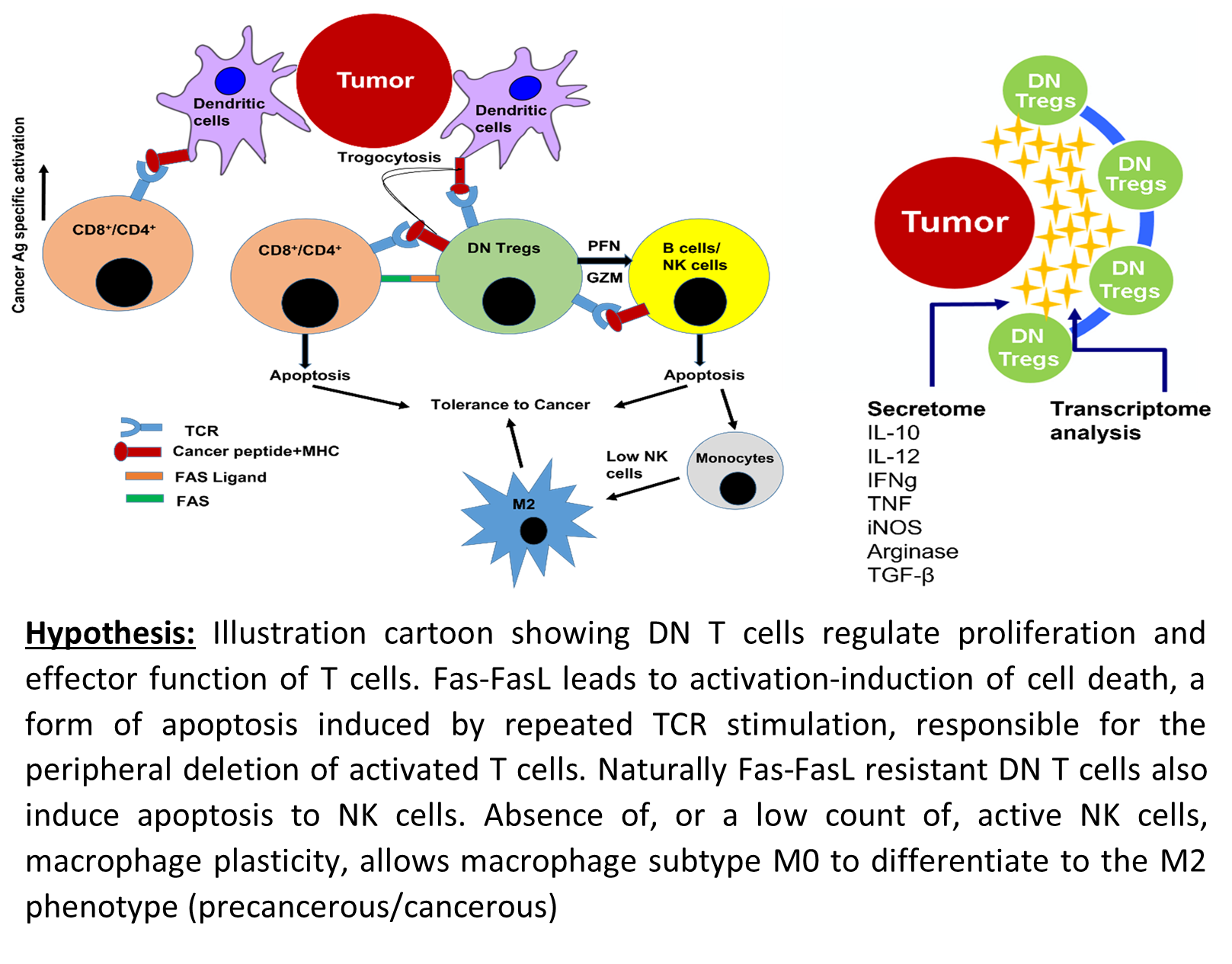
Lymphocytic profiling in thyroid cancer provides clues for failure of tumor immunity
Thyroid Cancer Screening Using Tumor-Associated DN T Cells as Immunogenomic Markers
MQ-NK cell cross talk: We are also studying the role of surveillance innate immune-cells like natural killer (NK) cells and macrophages (MQ), which are complimentary to each other in their actions. Our lab is studying the molecular basis of macrophage-NK cell interface in Thyroid Cancer, Euthyroid Hashimoto (ETH) and Graves (GD). This study will open new vistas for immunopathology and therapeutic intervention in thyroid autoimmunity and thyroid cancer.
Role of innate immune system in breaking the cancer tolerance: We bring the recent advances in understanding the tumor microenvironment and immunity in thyroid cancer. The heterogeneity of the immune responses in tumor microenvironment influences cancer development and suppression, and its outcome. Immunotherapies targeted to crosstalk between macrophages and NK cells may be affordable for patients with incurable anaplastic cancers.
NK cells affect the phenotypic and functional behavior of macrophage subsets, and in turn the macrophages impact the cytotoxic and cytokine secretory potential of NK cells. This cellular crosstalk has implication in the outcome of various autoimmune diseases and cancers. Flagellin is emerging as a potent immune modulator, shaping both the innate and adaptive arms of immunity. Here, we want to explore how flagellin modulates the Macrophage-NK cell cross talk. Our aim is to explore the use of flagellin as potent anti-tumor agent in thyroid cancers.
Bacterial flagellin—a potent immunomodulatory agent



