Faculty: Steven J. Sucheck, Ph.D.

Professor and Chair
Email: steve.sucheck@utoledo.edu
Office: WO 3276
Phone: (419) 530-1504
Fax: (419) 530-1990
Professional Background:
B.S., 1992, Univ. of Toledo
Ph.D., 1998, Univ. of Virginia
NIH Postdoctoral Fellow, 1998-2000, The Scripps Research Institute
2000-2002 Sr. Scientist, Optimer Pharmaceuticals;
2003-2005 Group Leader, Optimer Pharmaceuticals
Research Synopsis:
The goals of our bioorganic chemistry program are to design and synthesize small molecules and small molecule probes with desired biological activities and understand their structure activity relationships and target interactions.
We currently have three NIH funded programs. The first program includes the synthesis and study of enzyme inhibitors in the trehalose utilization pathways of Mycobacterium tuberculosis (Mtb). Mtb is the infectious etiological agent of tuberculosis (TB). Mtb is estimated to infect up to one third of the world’s population, of those people about eight million develop an active infection. About 1.4 million people die of TB every year. Further, extensively drug resistant strains of Mtb have emerged making many cases of TB difficult, if not impossible, to treat. Thus, there is an urgent need to discover small molecule probes that can be used to study or identify essential enzymes in Mtb. Our studies may lead to therapies to treat TB. Specifically we are discovering and evaluating new covalent and non-covalent glycoside hydrolase and esterase inhibitors.
A second exploratory program is related to the synthesis of Mtb-active marine natural products which show improved selective activity against non-replicating Mtb. The new derivatives can also be used to generate chemical probes which will be used in combination with chemical biology methods for the identification of new biological targets. These studies are anticipated to be useful for identifying next generation anti-tubercular agents.
The third program involves the use of antibody recruiting molecules (ARMs) to generate improved immunotherapeutics. Vaccines can be improved by directing antigens via the use of ARMs and natural antibodies to antigen presenting cells (APCs). We have used this approach to improve antibody and T-cell responses against tumor associated-antigens (TAA) in mice. We are currently working to demonstrate this effect for other antigens such as bacterial antigen. A second aspect of this program is the development of methods for vaccine and antigen synthesis.
These programs are highly collaborative and allow students interactions with structural biologists, microbiologists, immunologists, and natural product experts.
Program 1. Synthesis of chemical probes to investigate enzymes involved in trehalose utilization.
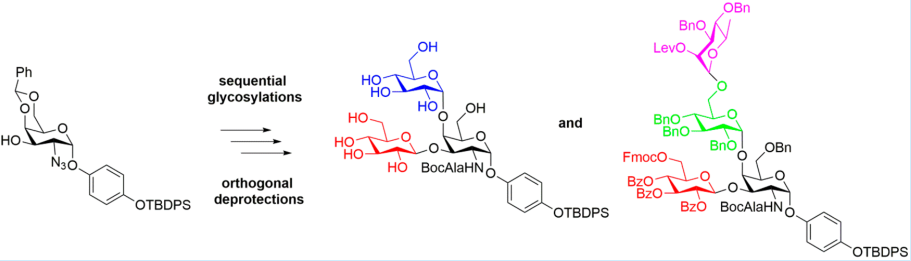
Trehalose is a disaccharide and an essential metabolite found in Mtb. Several enzymes involved in the utilization of trehalose have been found to be essential to the organism’s survival. We are developing probe molecules which can be used to understand the detailed molecular interactions that occur between the enzymes involved in trehalose utilization and their substrates. For example, we are using carbohydrates as components of substrate analogues and transition state analogues of Mtb Antigen 85s (Ag85s). Ag85s are mycolyl transferases responsible for the synthesis of the virulence factor trehalose dimycolate (TDM). Ag85s are also involved in mycolation of carbohydrates on the cell wall. Collectively, this process can be thought of as mycolyl membrane maintenance. Since Ag85s act on ß-D-arabinofuranoside- and trehalose-based cell wall structures these carbohydrates are prominent in our inhibitor designs. The compounds resulting from these investigations are believed to be useful for understanding key steps in the bacterial cell wall synthesis of Mtb. Since the bacterial cell wall is a well-established target for a number of anti-bacterial therapies these studies may potentially lead to an improved understanding of cell wall synthesis targets or to new antibiotics useful for treating the growing threat of multi-drug resistant Mtb. In addition to Ag85s, we are exploring essential enzymes such as GlgE, a glycoside hydrolase-like phoshorylase. GlgE is one enzyme responsible for α-glucan synthesis. It has been reported that absence of GlgE leads to self-poisoning by the accumulation of phosphosugar maltose-1-phosphate (M1P), directed by a self-amplifying feedback response leading to cell death. GlgE is essential for survival of the pathogen, and the absence of a human homologue substantiates GlgE as a new drug target. Figure 1 shows some of the roles of trehalose in Mtb. In addition to these targets we are designing inhibitors against other essential enzymes in the trehalose utilization pathway. For more information see: http://www.utoledo.edu/offices/marketing/utnews/pdfs/UTnewspdfs/2014.03.17.UTNews.pdf
Figure 1. Trehalose utilization pathways
Program 2. Synthesis of marine natural products active on dormant Mtb.
TB is a tremendous global health threat with nearly 480,000 new cases of multidrug-resistance TB (MDR-TB)reported in 2015. TB is difficult to treat and control in part due to its capacity to enter a non-replicating ‘dormant’ state. It is estimated that billons of individuals harbor a latent Mycobacterium tuberculosis (Mtb) infection, which causes no symptoms and can last a lifetime. One of the most significant barriers to treating Mtb is the intrinsic drug resistance of dormant bacilli. To overcome this barrier we are synthesizing new terpenoid-based natural products known to act on dormant Mtb.
Program 3. Targeting APCs to generate improved immunotherapeutics.
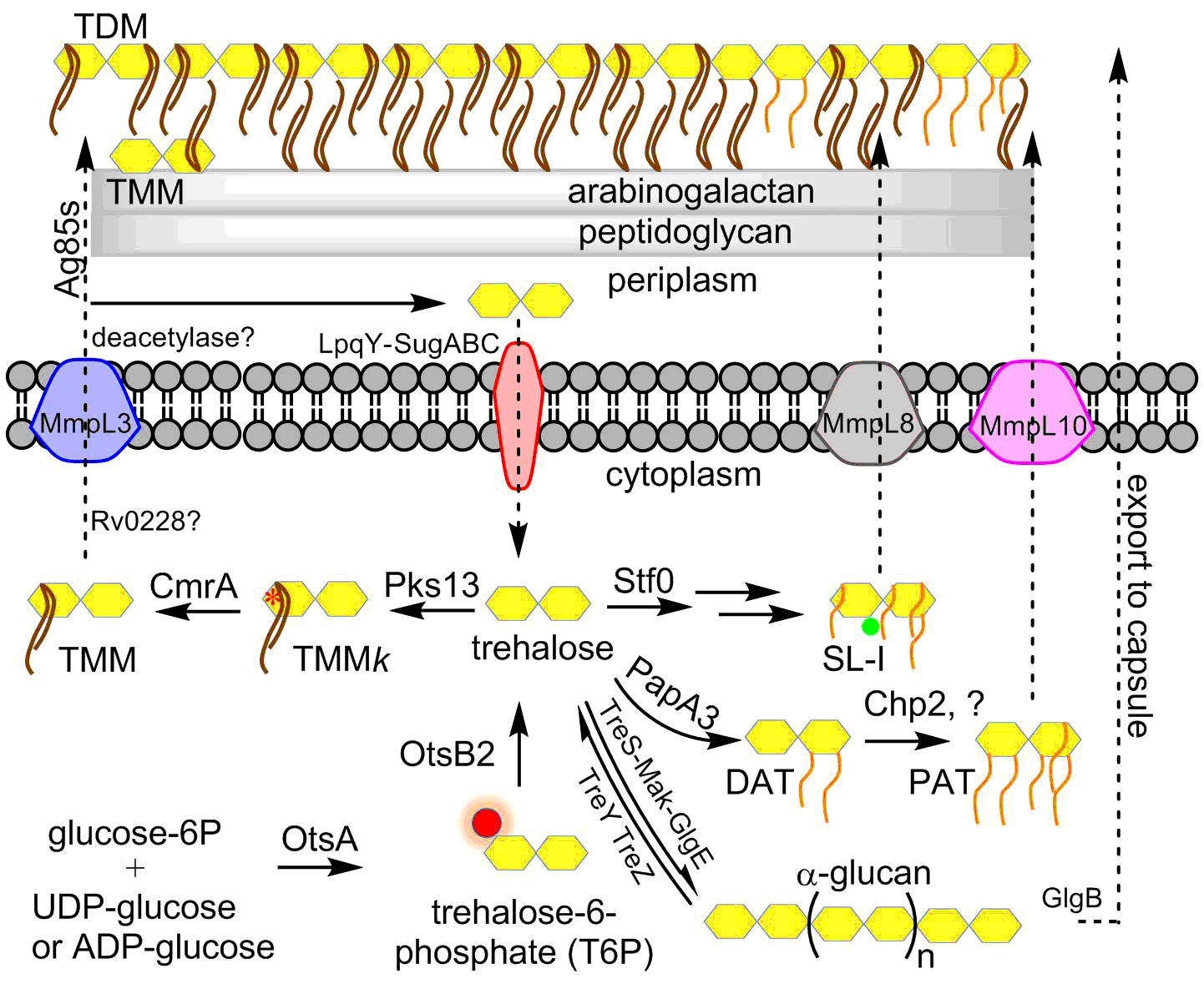
Carbohydrates found as naturally occurring glycoconjugates frequently serve as important markers for cancer and are potential targets for active immune therapy. One example is the human epithelial type 1 mucin (MUC1), a large polymorphic transmembrane mucin, expressed on normal glandular epithelia. In cancer cells the MUC1 glycoform distribution changes to reduced glycosylation with many of the glycan chains truncated relative to normal cells exposing hidden glycan epitopes on the molecule. Together these aberrant structures are referred to as tumor-associated antigens (TAAs). Individuals with early breast cancer who possess natural anti-MUC1 antibodies have been shown to have a reduced likelihood of distant metastases and better disease-specific survival. We are interested in synthesizing homogenous MUC1 glycopeptides which contain TAA epitopes. We are also exploring the use endogenous anti-L-rhamnose antibodies found in humans to augment immunogenicity of cancer vaccines by introducing antibody recruiting molecules (ARMs) in to vaccine designs, see Figure 2 for an illustration of the concept. For more information see: http://utnews.utoledo.edu/index.php/06_02_2011/researchers-integrate-disciplines-to-develop-cancer-vaccines
Figure 2. A liposomal anti-tumor immunotherapeutic (ARM groups in red)
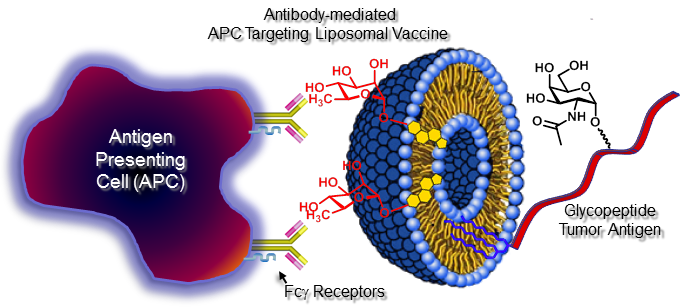
In addition, we have prepared antigens related to P. aeruginosa Figure 3, a difficult to treat pathogen that is responsible for many hospital acquired infections and deaths. These antigens along with others are being introduced into our vaccine platform
Figure 3. Synthesis of a tetrasaccharide component of the lipopolysaccharide of P. aeruginosa.