Argon

What happens if you make ice out of Argon?
Contributor: University of Toledo Instrumentation Center
About the Display: As one of the noble gases, Argon was chosen by the Instrumentation Center. The display box features a discharge tube in the shape of the element's symbol. The tube is filled with Argon and powered by a transformer. When an electric charge excites the Argon gas in the tube, the Argon emits a blue glow. The glow comes from an electron of the gas becoming excited from the energy. The excited electron leaves its electron shell and orbits the nucleus of the atom at a higher state. Eventually, the electron will return to its original state, releasing the extra energy as light.
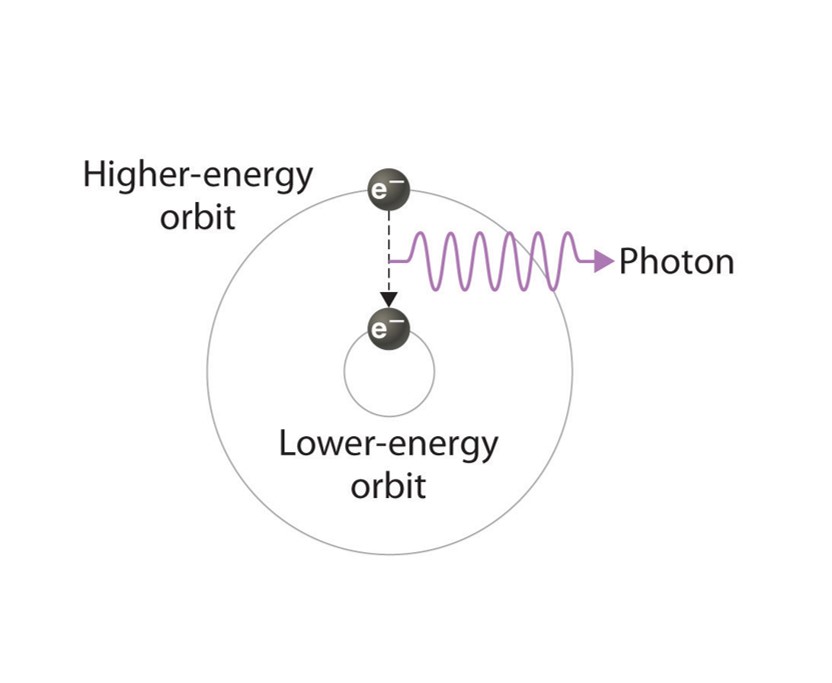
Above: A basic illustration of how an excited electron returns to its regular state and emits light. Picture courtesy of the open textbook library
Back to the Periodic Table
Symbol: Ar
Atomic Number: 18
Atomic Mass: 39.948 u
Electron Configuration: [Ne] 3s23p6
Year Discovered: 1894
Discovered By: Lord Rayleigh and Sir William Ramsay


