Nitric Acid Disposal Alert
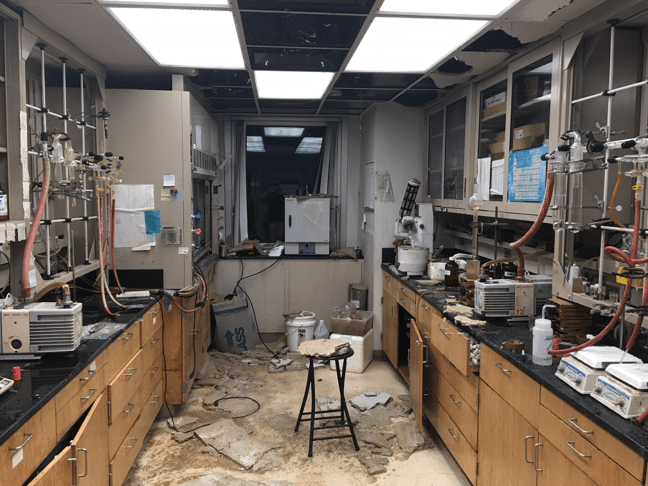
The University of Toledo has experienced waste bottle explosions related to the disposal and management of nitric acid waste.
This is the most common cause of laboratory waste bottle explosions that EHRS has encountered.
September, 2024: A glass bottle containing Hydrochloric Acid, Nitric Acid and Acetone exploded in a laboratory on campus. The explosion was violent enough that it was heard and felt in offices upstairs.
January, 2019: EHRS responded to a late-night laboratory fire which activated the sprinkler system and flooded multiple laboratories across multiple floors of the building. The root cause was thought to be use of a defective hot plate - or - addition of organic wastes to a bottle containing nitric acid resulting in a delayed explosion/fire.
Numerous other Universities have reported similar explosive incidents.
Nitric acid reacts violently with many materials including organics (alcohols, acetone, acetic acid), reducing agents (metal hydrides, formic acid, phosphorous acid) and metals (lead, zinc, aluminum).
Care should also be taken when preparing or disposing of Aqua Regia (hydrochloric acid and concentrated nitric acid). Aqua Regia produces several gases including Nitrosyl chloride, Chlorine and Nitric oxide. If closed in a bottle – this mixture may also explode.
Of particular concern: Mixtures of nitric acid and organics often produce a delayed reaction. It may take several minutes, hours or even days for a violent reaction to occur. This reaction evolves carbon dioxide, heat and nitrogen oxides which will overpressure and explode sealed waste bottles – violently – damaging equipment, throwing glass and showering the area in acid.


