Carbon

Contributor: Cole and Koen
About the Display: The display has various allotropes of carbon and items containing carbon:
- Amorphous Carbon - The charcoal in the display was made in our woodburning stove. Charcoal is mostly made up of amorphous carbon and is created when wood is baked in a high heat and low oxygen environment.
- Graphite - Graphite is in the display because it is made from layers of carbon atoms. The layers of carbon cause it to flake apart and be soft, unlike diamonds. Graphite is commonly found in pencils and is often incorrectly called lead when referred to as a "lead pencil".
- Diamond - Diamonds are like graphite because they are made from carbon. However, the carbon atoms are arranged differently than graphite which makes it a different color and extremely hard.
- Human Body - A picture of Cole is also included. Although not an allotrope, Cole (the human body), is made up of 18% carbon.
 About the Contributor: About Cole - I am eleven years old and I play soccer. When my dad told me I'm partly
made of carbon, I decided that I should use carbon as my element.
About the Contributor: About Cole - I am eleven years old and I play soccer. When my dad told me I'm partly
made of carbon, I decided that I should use carbon as my element.
About Koen - I am nine years old. I like to read and I love to play.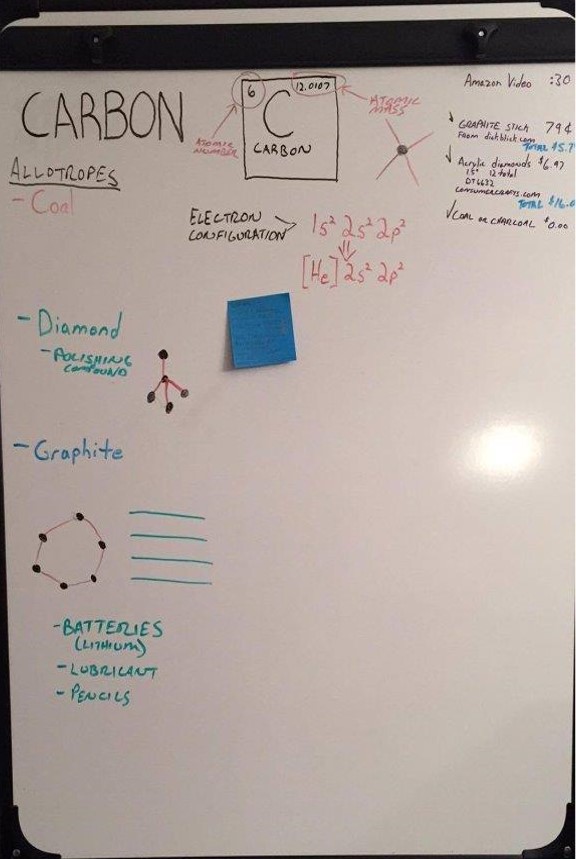
Cole and Koen are home-schooled by their mother. For this project, they added extra time to their science curriculum to study carbon!
Back to the Periodic Table
Symbol: C
Atomic Number: 6
Atomic Mass: 12.0107 u
Electron Configuration: [He] 2s22p2
Year Discovered: Approximately 3750 BCE/ Recognized as an element in 1789
Discovered By: The Egyptians and Sumerians/Antoine Lavoisier

