Iodine
Contributor: Joe Meyer
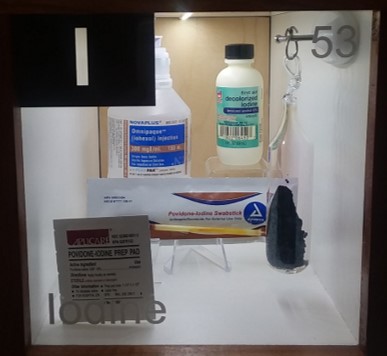
About the Display:
- An ampule with crystalline iodine. There is an ongoing experiment happening here! Upon heating from the LED lights (and at normal pressure) iodine goes directly from the solid phase into the gas phase - skipping the usual liquid phase. If you look carefully you can see the violet gas phase in this closed ampule. Gas phase iodine crystallizes back into the solid phase at the cooler end of the ampule, the one facing you and away from the LEDs. What happens when we turn off the lights in the night?
- A bottle of a CT contrast agent containing iohexol. The organic iodine compound adds contrast to fluids and body parts by blocking the X-rays passing through your body. The body parts that contain iohexol are then clearly separated in contrast to the one that do not contain iodine. It is a non-ionic water soluble drug, which was approved for X-ray imaging in 1985. For other contrast agents see gadolinium and barium.
 Pictured: the iohexol molecule
Pictured: the iohexol molecule
- First aid iodine. Iodine is an antibacterial agent and disinfectant. It was discovered in 1811 and extensively used during the civil war to treat soldiers.
- Iodine Swabstick. These cotton swabs contain iodoprovidone used as an antiseptic. The complex released the intensely colored iodine which disinfects and also colors your skin.
 About the Contributor: Joe Meyer is a graduate from the University of Toledo with a degree in Exercise Science.
He also has a strong background in pharmaceutical science.
About the Contributor: Joe Meyer is a graduate from the University of Toledo with a degree in Exercise Science.
He also has a strong background in pharmaceutical science.
Back to the Periodic Table
| <Previous element-| | |-Onward to next element!> |
Symbol: I
Atomic Number: 53
Atomic Mass: 126.90447 u
Electron Configuration: [Kr] 4d105s25p5
Year Discovered: 1811
Discovered By: Barnard Courtois


